
Vivolight Medical Receives FDA 510(k) Clearance for OCT System, Accelerating Global Expansion
April 11, 2025, Vivolight Medical announced that its independently developed Optical Coherence Tomography (OCT) system has obtained FDA 510(k) clearance. This milestone not only enbale the U.S. market entry, but also streamlines regulatory pathways across 20+ countries recognizing FDA approvals, significantly advancing the company's global commercialization strategy .
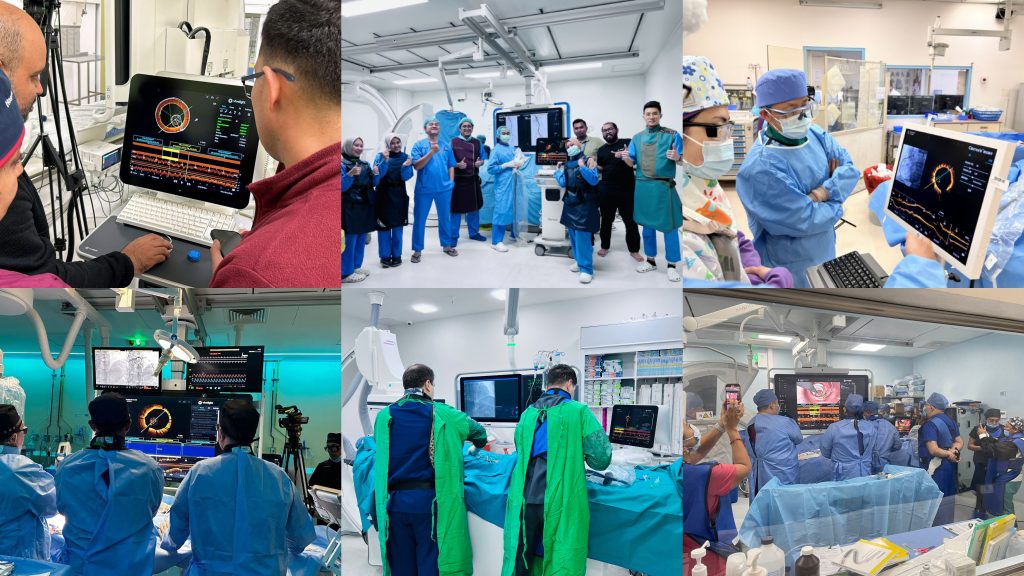
Since 2023, Vivolight has became the first Chinese OCT manufacturer to enter into international markets. Until now, the OCT system has been implemented hospitals acrtoss 8 countries and regions that accept China's NMPA registration, with over 50,000 clinical cases performed globally (including China). This achievement underscores China's growing influence on high-end medical device innovation .
The FDA clearance provides immediate access to:
Compared to angiography, OCT reduces procedural uncertainties by providing layered arterial views previously unavailable in clinical practice.
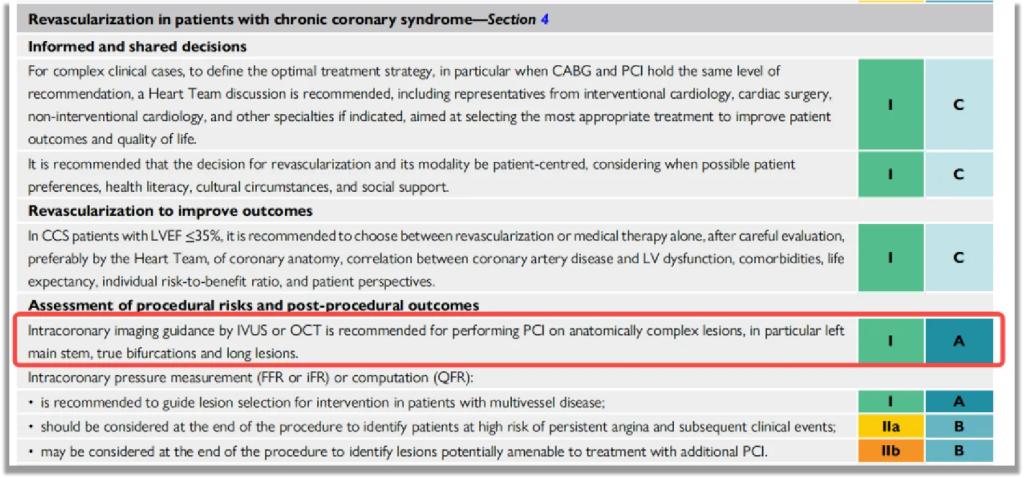
The 2024 coronary intervention guidelines elevated OCT to Class I (Level A) recommendation alongside IVUS and FFR, validating its:
• Proven outcomes in complex PCI
• Key role in stent optimization and complication prevention
This positions OCT for rapid adoption as a gold-standard imaging modality.
Guided by the vision of “Precision Imaging, Intelligent Intervention,” Vivolight Medical is committed to transforming the landscape of intravascular imaging through cutting-edge innovation. The FDA 510(k) clearance marks a pivotal achievement in the company’s regulatory and commercialization journey, furthering its commitment to delivering world-class imaging tools to interventional cardiologists and patients around the world.
With this regulatory achievement, Vivolight accelerates its vision of intelligent intervention through advanced imaging – bringing safer, more effective PCI to global markets.
Leave A Message
Scan to WhatsApp :
