
Vivolight Medical Highlights Multimodal Cardiovascular Imaging Innovation at EuroPCR 2025
EuroPCR 2025 concluded with a wave of technological breakthroughs, and Vivolight Medical stood at the forefront with its revolutionary P80 Multimodal Cardiovascular OCT System. As the world’s first FDA-cleared OCT platform featuring integrated AI diagnosis, the P80 attracted global interest and set new standards in cardiovascular imaging.
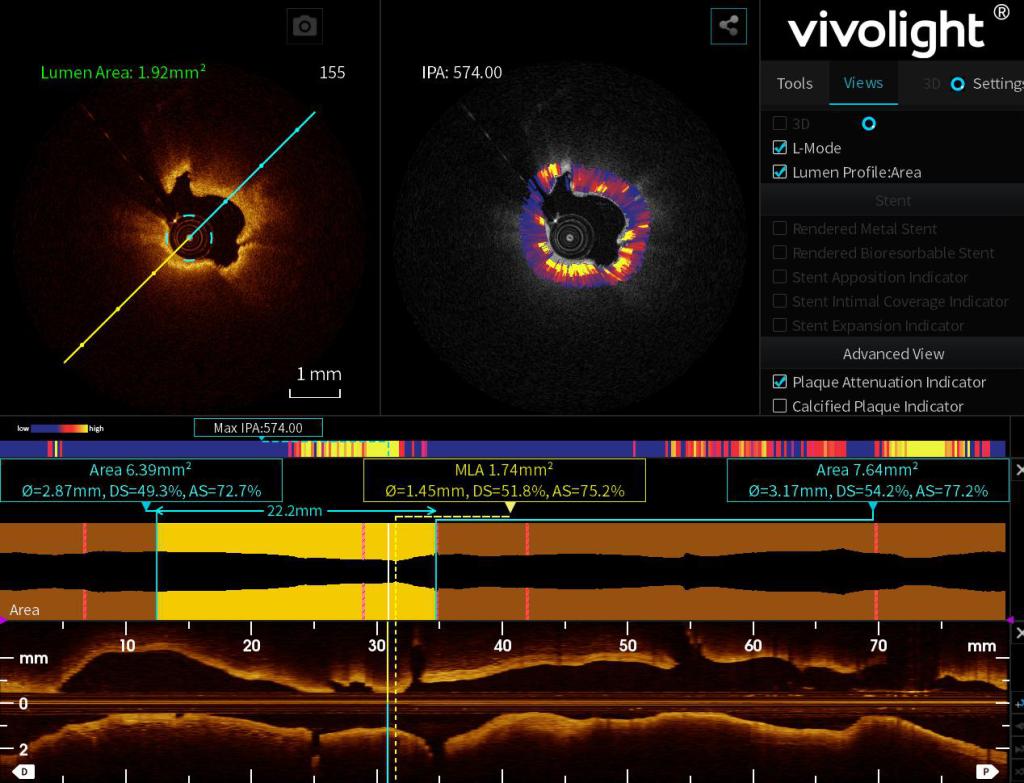
At the heart of Vivolight’s showcase was the P80,a next-generation multimodal OCT system, delivers a One-Stop Diagnostic Workflow. Physicians and industry experts from across the globe were impressed by the platform’s quantitative precision, enhanced image clarity, and seamless integration of AI-guided insights.

EuroPCR 2025 became a powerful validation of Vivolight’s global positioning. Delegates and agents from regions including Chile, Dubai, Brazil, and Panama actively explored strategic collaborations—reinforcing the international demand for advanced OCT solutions in cardiovascular care.
Even senior representatives from global MedTech giants visited Vivolight’s booth, highlighting China’s emerging leadership in high-end diagnostic technologies.
As interventional cardiology embraces digital transformation, Vivolight’s AI-powered imaging technology equips clinicians with actionable, real-time data to make faster and more accurate decisions. With integrated OCT-FFR, ICA, and IPA, the system delivers comprehensive insights in one platform—saving time and improving efficiency.
Vivolight Medical’s successful showing at EuroPCR 2025 signals the start of a new chapter in global cardiovascular innovation. Stay tuned for more expert interviews, IPA deep dives, and case-based content designed to empower frontline clinicians and advance precision medicine.

Leave A Message
Scan to WhatsApp :
