
San Francisco, October 2025 — Transcatheter Cardiovascular Therapeutics (TCT) is one of the world’s leading conferences dedicated to interventional cardiology. The 37th edition of TCT will take place at the Moscone Convention Center in San Francisco from October 25 to 28, 2025. Bringing together more than 10,000 attendees—including over 5,000 physicians—and featuring a bustling exhibition of over 100 companies from more than 100 countries, TCT remains a premier global platform where clinicians, researchers, and industry leaders exchange breakthrough data and innovations.
Vivolight’s First Appearance at TCT
Vivolight marks a significant milestone in expanding its presence in the U.S. and advancing its global footprint in cardiovascular imaging. As a pioneer in optical coherence tomography (OCT) imaging in China, the company is leveraging this international platform to showcase its latest cardiovascular imaging technologies and engage with clinicians, researchers, and partners worldwide.

Regulatory Milestone
On April 11, 2025, the U.S. FDA granted 510(k) clearance (K242098) for Vivolight Medical’s Cornaris P80-E and Cornaris Mobile-E OCT Imaging Systems, along with the LumenCross F2 Imaging Catheter. This clearance authorizes U.S. commercialization and validates the products’ compliance with rigorous regulatory standards.
Vivolight’s OCT imaging systems have already been adopted by more than 300 hospitals throughout China, earning widespread clinical recognition for their image quality, reliability, and ease of use. The Cornaris P80-E and Cornaris Mobile-E systems deliver high-resolution, stable imaging performance comparable to that of leading global brands.
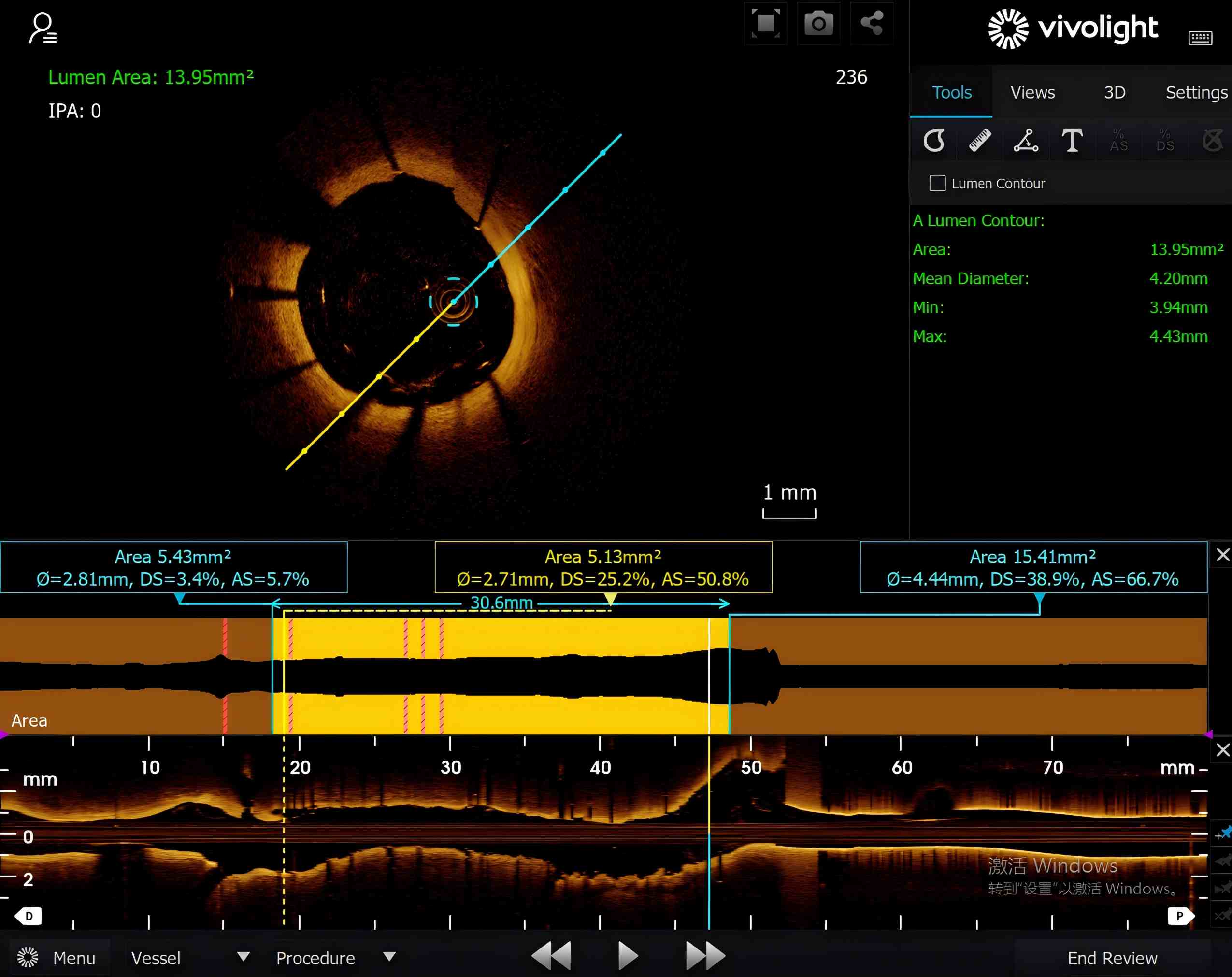
Vivolight’s products have entered markets including Indonesia, Chile, Armenia, Hong Kong, and Macao, marking steady progress in the company’s internationalization. Guided by continuous innovation and clinical collaboration, Vivolight is committed to advancing intravascular imaging technologies that empower physicians and improve cardiovascular care worldwide.
Conclusion
Vivolight Medical is a high-tech medical device company specializing in optical imaging technologies for cardiovascular intervention. Through it's independent R&D, clinical collaboration, and AI-driven innovation, Vivolight continues to push the boundaries of OCT imaging—delivering solutions that enhance precision, efficiency, and patient outcomes in interventional cardiology.
Leave A Message
Scan to WhatsApp :
